James Clerk Maxwell - The Great Unknown
Kevin Johnson
Kinetic Theory of Gases
One of Maxwell's major contributions to the scientific world was to provide a mathematical basis for the kinetic theory of gases. This was a subject he had become interested in whilst studying the motion of gases in Saturn's rings. He developed work by a German physicist, Rudolf Clausius who built on the basic atomic theory that a gas consisted of flying molecules with velocities that were dependant on pressure.
Clausius explained why gases with particles that should have travelled at speeds up to several thousands of meters per second, would move far slower in reality. He showed that the molecules would collide with each other and developed the idea of the mean-free-path as a measurement of the average distance travelled by a molecule between collisions. In 1859 Clausius published a paper giving a calculation for the mean-free-path in terms of the average distance between molecules and the distance between the centres of colliding molecules at impact.
At this time the common conception had been that all the molecules in a gas travelled at the same speed but Maxwell noticed that these collisions would result in particles having different speeds. He realised that to advance in this area it was necessary to calculate the speeds of different molecules. Maxwell achieved this by creating the formula that is now known as Maxwell's Distribution :
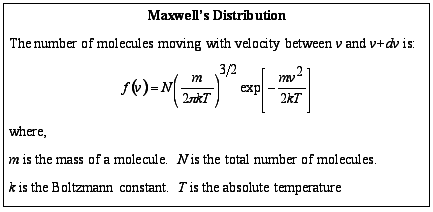
[It should be noted that much of this work had already been completed by J.J. Waterston between 1846 and 1851 but remained unnoticed until 1892.]
This was ground-breaking as it was the first time the matter had been considered to be probabilistic. Austrian physicist, Ludwig Boltzmann subsequently modified it, in 1868, to explain heat conduction, producing the Maxwell-Boltzmann Distribution Law.
This work was presented in Maxwell's paper Illustrations of the Dynamic Theory of Gases in which he also considered a combination of two types of particles and the relation needed between the average velocities for the state to be steady. He gave calculations of the velocity, mean-free-path, and number of collisions of the molecules in the mixture at a given temperature. He also gave the first accurate expression of the pressure of a gas assuming random molecular speeds and showed it to be the same as what had previously been obtained on the assumption of uniform speeds. In addition he repeated, more accurately, Waterston's deduction of Avogadro's law, that equal volumes of gas, at equal temperatures and pressures contain equal numbers of molecules.
These ideas were refined and reproduced in a more advanced paper On the Dynamical Theory of Gases in 1867. He corrected several errors in his 1859 paper for which he acknowledged Clausius:
Using this information, he tied together all the theory on the subject, which had been in some state of disarray, introducing concepts such as relaxation time to explain the diffusion of stress in fluids. This provided a solid theoretical grounding for the kinetic theory of gases that had never before been in place. He also explained that the viscosity of a gas should be independent of its density an unexpected result as common sense seemed to indicate the opposite. He had first calculated this in 1860 but it was difficult to believe until he and his wife, Katherine, proved it experimentally in 1865.
Maxwell continued to work sporadically on the kinetic theory of gases for the rest of his career. In 1870 he published his book The Theory of Heat in which he created Maxwell's Demon, a thought experiment designed to contradict the second law of thermodynamics. This problem took 70 years to solve and also stimulated research in information technology. He also wrote a paper On Boltzmann's Theorem on the Average Distribution of Energy in a System of Material Points in which he introduced the idea of ergodic hypothesis which says in Maxwell's words:
This was an excellent piece of work that laid the foundations of statistical mechanics.
He then wrote a paper On Stresses in Rarefied Gases Arising from Inequalities of Temperature in which he developed the theory of rarefied gases to account for observations. This began an entirely new branch of physics that eventually enabled us to understand the upper atmosphere and the fringes of space.
So it is clear Maxwell's influence in this area was great. His 1867 paper was magnificent and at the time was considered to be his greatest piece of work. He provided many great insights into the subject and was described by Peter Guthrie Tait as "the leading molecular scientist" [3, p1] of his day.
Clausius explained why gases with particles that should have travelled at speeds up to several thousands of meters per second, would move far slower in reality. He showed that the molecules would collide with each other and developed the idea of the mean-free-path as a measurement of the average distance travelled by a molecule between collisions. In 1859 Clausius published a paper giving a calculation for the mean-free-path in terms of the average distance between molecules and the distance between the centres of colliding molecules at impact.
At this time the common conception had been that all the molecules in a gas travelled at the same speed but Maxwell noticed that these collisions would result in particles having different speeds. He realised that to advance in this area it was necessary to calculate the speeds of different molecules. Maxwell achieved this by creating the formula that is now known as Maxwell's Distribution :

[It should be noted that much of this work had already been completed by J.J. Waterston between 1846 and 1851 but remained unnoticed until 1892.]
This was ground-breaking as it was the first time the matter had been considered to be probabilistic. Austrian physicist, Ludwig Boltzmann subsequently modified it, in 1868, to explain heat conduction, producing the Maxwell-Boltzmann Distribution Law.
This work was presented in Maxwell's paper Illustrations of the Dynamic Theory of Gases in which he also considered a combination of two types of particles and the relation needed between the average velocities for the state to be steady. He gave calculations of the velocity, mean-free-path, and number of collisions of the molecules in the mixture at a given temperature. He also gave the first accurate expression of the pressure of a gas assuming random molecular speeds and showed it to be the same as what had previously been obtained on the assumption of uniform speeds. In addition he repeated, more accurately, Waterston's deduction of Avogadro's law, that equal volumes of gas, at equal temperatures and pressures contain equal numbers of molecules.
These ideas were refined and reproduced in a more advanced paper On the Dynamical Theory of Gases in 1867. He corrected several errors in his 1859 paper for which he acknowledged Clausius:
It is to Professor Clausius of Zurich, that we owe the most complete dynamical theory of gases ... there were several errors in my theory of conduction of heat in gases which M. Clausius has pointed out in an elaborate memoir on the subject. [10, p139-140]
Using this information, he tied together all the theory on the subject, which had been in some state of disarray, introducing concepts such as relaxation time to explain the diffusion of stress in fluids. This provided a solid theoretical grounding for the kinetic theory of gases that had never before been in place. He also explained that the viscosity of a gas should be independent of its density an unexpected result as common sense seemed to indicate the opposite. He had first calculated this in 1860 but it was difficult to believe until he and his wife, Katherine, proved it experimentally in 1865.
Maxwell continued to work sporadically on the kinetic theory of gases for the rest of his career. In 1870 he published his book The Theory of Heat in which he created Maxwell's Demon, a thought experiment designed to contradict the second law of thermodynamics. This problem took 70 years to solve and also stimulated research in information technology. He also wrote a paper On Boltzmann's Theorem on the Average Distribution of Energy in a System of Material Points in which he introduced the idea of ergodic hypothesis which says in Maxwell's words:
The system left to itself in its actual state of motion will, sooner or later, pass through every actual phase which is consistent with the equation of energy [10, p151]
This was an excellent piece of work that laid the foundations of statistical mechanics.
He then wrote a paper On Stresses in Rarefied Gases Arising from Inequalities of Temperature in which he developed the theory of rarefied gases to account for observations. This began an entirely new branch of physics that eventually enabled us to understand the upper atmosphere and the fringes of space.
So it is clear Maxwell's influence in this area was great. His 1867 paper was magnificent and at the time was considered to be his greatest piece of work. He provided many great insights into the subject and was described by Peter Guthrie Tait as "the leading molecular scientist" [3, p1] of his day.